Abstract
Introduction
Emicizumab is a recombinant, humanized bispecific monoclonal antibody that mimics the function of factor VIII (FVIII) which results in a significant reduction in the annualized bleeding rate in patients with hemophilia A (HA), however, the degree with which emicizumab corrects the coagulation defect remains unclear. The objective of this study was to compare the current available laboratory methods in clinical practice; one-stage clotting factor assays (OSCA), bovine and human chromogenic FVIII activity (bovCHR and humCHR, respectively) and FVIII Equivalency of Emicizumab by Thrombin Generation (F8EmT).
Aims
The aim of this study is to address the differences of FVIII activity with different techniques in patients with severe HA with inhibitors on emicizumab.
Materials and Methods
Factor VIII levels are determined with an activated partial thromboplastin time (aPTT), OSCA using SynthASil on the ACL TOP 500 (Instrumentation Laboratory, Bedford, MA). Factor VIII activity is also determined photometrically via the Chromogenix Coatest® SP4 FVIII chromogenic assay kit (bovCHR, Diapharma Group, West Chester, OH) and the Biophen FVIII:C chromogenic assay kit (humCHR, Aniara Diagnostica, West Chester, OH). For F8EmT, linear regression was utilized to model the FVIII levels as a function of the endogenous thrombin potential (ETP) and peak thrombin values for patients with mild/moderate hemophilia. Then, we used the ETP and peak thrombin results of the severe HA patients on emicizumab with the calibration curve to calculate their F8EmT. Association between patient weight and their F8EmT were also examined and evaluated by linear regression.
Results
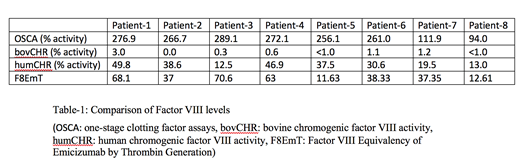
Data is presented for eight patients with severe HA with inhibitors on emicizumab in the non-bleeding state (Table-1). All patients' FVIII levels measured with OSCA are in or above the normal range (94.0-289.1). Bovine chromogenic FVIII activity is in the severe hemophilia range for five out of eight patients, for the rest it is in the moderate hemophilia range. Human chromogenic FVIII activity ranged between 12.5-49.8%. Factor VIII Equivalency of Emicizumab by Thrombin Generation is either in the mild hemophilia or normal range in all participants of the study.
Conclusion
One-stage clotting factor assays demonstrated falsely high results as expected since it is activated partial thromboplastin time based. Bovine chromogenic FVIII activity results were consistent with the severe HA range of the patients though a few had results slightly above that level. Previous literature has stated that the humCHR in patients on emicizumab results in FVIII levels of ∼30% when emicizumab is at its therapeutic concentration (∼50 mcg/ml). This study also demonstrated similar results with 5/8 patients having levels 30-50%. F8EMT levels were mostly consistent with the humCHR. In conclusion, understanding the degree to which emicizumab corrects the coagulation defect of is an important goal as it has clinical implications.Certainly, additional studies with higher participant numbers are needed to confirm these findings.
Young: Apcintex, BioMarin, Genentech/Roche, Grifols, Novo Nordisk, Pfizer, Rani, Sanofi Genzyme, Spark, Takeda, and UniQure: Consultancy; Genentech/Roche, Grifols, and Takeda: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal